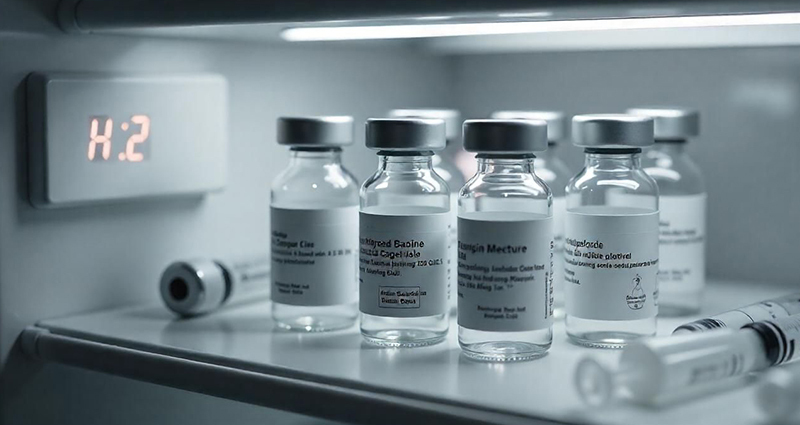
Proper storage of pharmaceuticals from research and development through dispensing is crucial to ensure that the products remain safe and effective. Temperature and humidity can have drastic effects on drugs and their ingredients. If stored at the wrong temperatures for even a short period of time, they can become ineffective, lose potency or even become harmful to patients.
With some candidate drugs costing thousands of dollars per dose, losing one drug or a batch of drugs could be catastrophic to research, development or a clinical trial. While many drugs are stable at room temperature, there is a growing number of drugs, particularly biologics, that must be kept refrigerated or frozen. Because of this, refrigerators and freezers need to be carefully monitored to ensure that the stock is always kept in appropriate and auditable environmental conditions.
Common Causes of Error
Human error, mechanical issues and power failure are among the top causes for concern in pharmaceutical research and development. Human error occurs when refrigerators and freezers are left unmonitored. Many times, the staff are too busy to record log files or may inadvertently leave a door ajar, causing the temperature of the refrigerator or freezer to rise above safe levels.
In addition to human error, mechanical and power failures can happen at any time, often without warning. A remote monitoring system is the most cost-effective way to ensure that you are immediately notified of issues, allowing you to quickly take action and protect the quality of pharmaceutical products.
Safeguarding Against Temperature Fluctuation
It’s important to carefully control environmental conditions wherever drugs are stored. Areas of concern include temperature, humidity, light, and sanitation. While regulatory bodies like the World Health Organization (WHO) don’t recommend specific storage equipment, they do provide guidelines for good storage practices for pharmaceuticals. The WHO recommendations include:
- Have the recorded temperature monitoring data readily available for review.
- Check the equipment used for monitoring at suitable predetermined intervals, and record and retain the results of such checks.
- Keep all monitoring data for at least the shelf-life of the stored material plus one year.
- When mapping temperature, show the uniformity of the temperature across the freezer or refrigerator.
- Regularly check and calibrate equipment used for monitoring.
Pharmaceutical remote monitoring systems like Sensaphone’s Sentinel help you verify that everything is stored at the correct temperature. The system continuously monitors your environment and immediately notifies you if temperatures fall out of the preset range. It will send an alert to your predetermined contact list via email, phone call or text message.
In addition to temperature, threats like power failure and equipment failure can lead to the catastrophic loss of pharmaceutical products. Remote monitoring systems can detect and send warnings about these conditions so that personnel can take action before it’s too late. In addition, they can also monitor:
- Water leaks
- Humidity changes
- Unauthorized access and break-ins
Remote monitoring systems protect your assets 24/7 and provide an audit trail documenting their correct storage. All data is stored remotely and can be accessed and downloaded easily by desktop, tablet or phone. When years of research and expensive pharmaceuticals can be easily compromised by human error or equipment failure, it’s critical to take every step possible to ensure its safety. To learn more about how you can use temperature monitoring to protect your pharmaceuticals, download our Temperature Monitoring Toolkit.