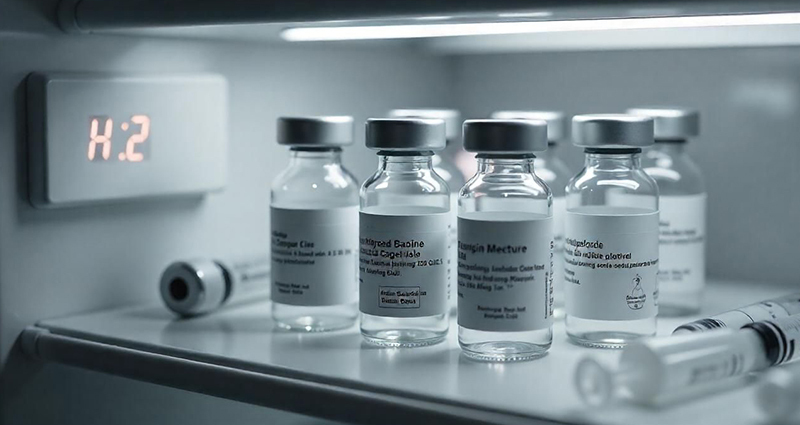
New COVID-19 vaccines from Pfizer and Moderna have brought to the public’s attention the importance and challenges of maintaining ultra-low temperatures for vaccines and other pharmaceuticals. Both vaccines have to be kept at very low temperatures and can be shipped and stored in a specialty freezer for up to six months. In addition, specialty freezers containing the Pfizer vaccine can’t be opened more than twice a day and need to be closed within one minute of opening. And once it’s thawed, the vaccine can only be stored in a refrigerator for five days.
Why do COVID-19 vaccines have to stay so cold?
The Pfizer and Moderna vaccines use messenger RNA (mRNA) to unlock a person’s immune defenses and start producing one particular coronavirus protein. That protein generates an immune response in that person, which their body “remembers” when they are exposed to the coronavirus in the future.
This mRNA technology is very new. On the plus side, mRNA can be made much faster than other vaccines. The downside is that it can be “really easily destroyed because there are many, many enzymes that will just break it apart, ” explained Margaret Liu, a vaccine researcher who specializes in genetic vaccines. To keep the mRNA from falling apart, it must be kept in a deep freeze.
The Pfizer and Moderna vaccines use different mRNA. The Moderna vaccine’s lipid nanoparticle properties and structure do not degenerate as quickly as temperatures rise.
Why is the Cold Chain so important?
Because vaccines are made of biomolecules that become unstable outside of their native environments, they can be ruined if they fall outside of their approved temperature range. At best, the wrong temperature causes them to lose potency, but in some cases, vaccines can become toxic when they aren’t stored correctly.
For vaccines that have government-regulated temperatures, like those in the CDC’s Vaccines For Children (VFC) program, it is necessary for vaccine providers to continually monitor their temperature and document it in order to demonstrate compliance. VFC providers must follow specific guidelines and prove that their vaccines never fell out of the recommended temperature range.
The “chain” refers to all the steps that a vaccine goes through along the way from its manufacture through shipping, delivery and storage until it is administered to the patient. The cold chain is generally a chain of medical freezers that the vaccine is stored in as it travels to the patient. This journey is very expensive—estimated to account for up to 80% of overall vaccination costs.
The new Pfizer vaccine must be stored at -70 °C, which is colder than winter in Antarctica. To accomplish that, the vaccine must be transported and stored with dry ice if it is not in an ultra-low-temperature medical freezer. This has been successfully done in the past, such as when the Ebola vaccine that also required ultra-cold storage was used in several African countries.
The Moderna vaccine remains stable at -20 °C, which is closer to a standard medical freezer. In addition, the Moderna vaccine can be kept in a standard medical refrigerator for about 30 days.
Preventing cold chain failures
Many facilities use remote monitoring systems to ensure that items stored in medical-grade refrigerators and freezers remain at the desired temperature without any fluctuation. Temperature sensors in the freezer constantly take temperature readings so the system can alert you when temperatures inside change for any reason.
The system functions as a digital data logger—automatically storing the data in a remote location that can be accessed at any time by any desktop, tablet or phone. This means that personnel no longer need to be physically present at the location where the freezers are located.
This protects vaccine providers against the biggest causes of cold chain failure—equipment failure, power failure and human error.
1. Equipment failure
Freezers need to be running constantly at the correct temperatures. Regular equipment maintenance helps you avoid expensive repairs, but what happens if an issue occurs in between scheduled maintenance visits?
A remote monitoring system uses temperature sensors placed inside a freezer to constantly check the temperature independently of any alarms the freezer may provide. In addition, they can also monitor the freezer’s alarm panel for freezer-identified failures. This data is logged and allows you to identify patterns and trends in equipment and environmental conditions and detect problems before they cause costly downtime.
2. Power failure
You must maintain a constant power supply to freezers to ensure that vaccines are stored at the optimal temperature. Power outages can break the cold chain, so remote monitoring systems notify you as soon as there’s a change in power status by text message, email or phone call. A battery backup system ensures continuous monitoring during any outage so you can review data logs from during the outage to determine if the vaccines were compromised.
3. Human error
Human error occurs when refrigerators and freezers are left unmonitored. The staff might have left a door ajar or been too busy to log temperatures for a period of time. Remote monitoring systems can minimize the risk of human error. Staff members will receive a notification as soon as temperatures change for any reason. These notifications are particularly useful if your facility will be left unattended for extended periods of time.Ensuring constant temperature
It is ideal to monitor both the alarm panel on the freezer/refrigerator and the actual temperature inside it using a temperature sensor.
Today’s more advanced systems also allow users to log both the highest and lowest temperatures over a set period of time — often a 24-hour period. This provides a snapshot to make sure the temps were within range that day. This feature is convenient when the person responsible for temperatures is not the same person designated to receive alarm notifications.
Without this device, you would need to check and record the minimum and maximum temperatures twice a day. You would also need to record the date, time, your name and any actions taken in the event of a temperature excursion. This amounts to significant time spent on maintaining up-to-date temperature records.
Keeping data records safe and accessible
Cloud-based remote monitoring systems automatically record temperatures at set time intervals and store all the data in the cloud for easy access. Some systems also have a mobile app for viewing temperatures remotely so you can view your data from anywhere at any time.
You can log data at user-customizable intervals, and you can log separate intervals when in an alarm state. The data is downloaded remotely through a website, either via a local network or the cloud, where it can be printed or exported as a .pdf, Excel spreadsheet or .csv.
NIST certification
To help customers who are responsible for storing vaccines, Sensaphone offers temperature sensors that carry Traceable Calibration Certificates. These certificates indicate that the sensors are traceable to standards provided by the National Institute of Standards and Technology (NIST), a U.S. Commerce Department agency. These certificates ensure that a laboratory or manufacturer is fully equipped to calibrate equipment to NIST standards and that products offered by that manufacturer match the NIST-maintained measurement criteria. If you have specific questions about which temperature sensor is right for your refrigerators and freezers, our support team is on hand to make a recommendation.
Vaccines are expensive and difficult to replace. If they are compromised while stored at a doctor’s office, hospital or clinic, the facility has to pay for the loss. They also face the risk of compromised patient health. Remote monitoring systems protect vaccines 24/7 and provide an audit trail for proving compliance. Remote monitoring systems require a greater initial investment but cost far less when factoring in labor costs of manual recording and the “costs” of financial risk of compromised vaccines. Contact one of our experts today to learn more or find a solution that meets your exact needs.
Wireless sensors
Wireless temperature sensors provide an easier solution when installing the sensors, especially if multiple freezers that are not in the same room will be monitored. Since the wireless sensors do not require being tethered to the remote monitoring system, they can easily be moved as inventory and requirements change. It is suggested that the wireless sensors be powered via a plugged-in power supply and the batteries be used as a backup. In the event of a power outage, the batteries in the sensor will keep it communicating with the remote monitoring system so that no data is lost during the outage.
For more information about how to move your network-based Sensaphone products into an easier to manage, more user-friendly cloud-based system, contact our technical sales support team.