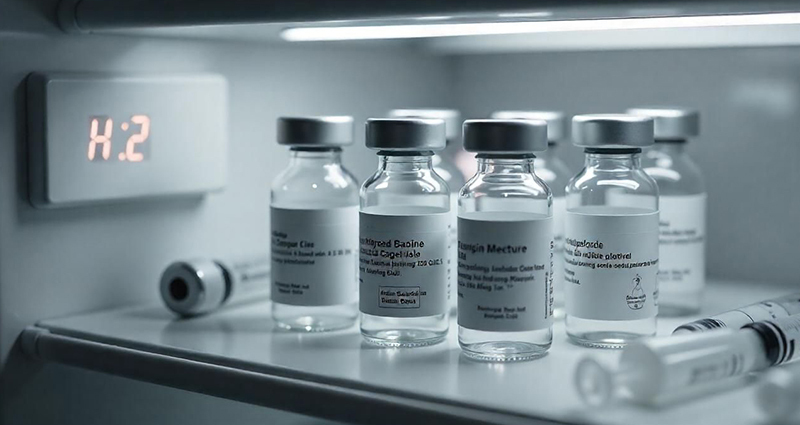
Injectable medications for weight loss and diabetes have seen a substantial increase in demand due to their effectiveness in managing these conditions. Precise refrigeration conditions are necessary to ensure stability, potency and safety of these pharmaceuticals. Remote monitoring systems are ideal for helping to maintain consistent Semaglutide storage temperature at medical offices, pharmacies and weight-loss clinics.
Growing usage of Semaglutide
Between 2016 and 2022, prescriptions for these medications rose by 221%, reflecting their rapid adoption across clinical and wellness settings. Sensaphone remote monitoring systems offer a reliable solution for ensuring proper Semaglutide storage temperature as well as other injectable treatments, helping pharmacies, doctors' offices and weight-loss clinics maintain drug efficacy and protect patients.
Like many critical vaccines, injectable medications require precise storage conditions to maintain stability, potency and safety. Each drug has its own temperature requirements and different formulations containing the same active ingredient may have distinct temperature specifications, both before and after first use.
For example, Semaglutide is the active ingredient in both Wegovy and Ozempic, yet each has unique storage guidelines. Failure to maintain proper Semaglutide storage temperature can degrade the drug, making it less effective.
Different Drug Temperature Requirements
While both Wegovy and Ozempic are GLP-1 receptor agonists containing Semaglutide, Wegovy is tailored for weight loss, while Ozempic is prescribed for managing type 2 diabetes. Each has specific refrigeration requirements.
- Ozempic should remain refrigerated until first use at 36 °F to 46 °F (2 °C to 8 °C). After first use, the pen can be stored for 56 days either at room temperature between 59 F° to 86 °F (15 °C to 30 °C) or in the refrigerator for 56 days. It must be discarded after this period.
- Wegovy should be stored in the refrigerator at 36 °F to 46 °F (2 °C to 8 °C) until first use, then kept at room temperature between 46 °F to 86 °F (8 °C to 30 °C) for up to 28 days.
Risks of Inexact Temperatures
As physicians, pharmacists and weight-loss clinics prescribe various forms of Semaglutide and Tirzepatide, proper storage becomes increasingly complex. Different formulations may require separate refrigerators with precise temperature controls. Even the same drug can have different storage requirements before and after initial use.
Manual temperature monitoring is time-consuming and prone to error, jeopardizing drug efficacy and regulatory compliance. Human error can occur if staff are too busy to record temperatures consistently or are unclear about the correct storage conditions for various injectable medications, leading to mistakes.
Failure to maintain exact temperatures can compromise medication potency. If a facility cannot verify that a drug was stored within the required temperature range, it may need to be discarded. Without accurate log files and documentation, the practice is responsible for losses. A refrigerator full of compromised injectables can result in thousands of dollars in wasted inventory delays in obtaining replacements, impacting patient care.
Automating Temperature Monitoring
Sensaphone remote monitoring systems provide a reliable, automated solution for consistent refrigeration temperature control of temperature-sensitive medications. Devices like the Sensaphone WSG30 wirelessly monitor Semaglutide storage temperature in medical-grade refrigerators and storage units across a range of -109 °F to 115 °F.
These systems automatically log temperature data and generate detailed reports for compliance and documentation. Real-time alerts notify designated personnel immediately if temperatures exceed safe thresholds, helping safeguard high-value medications.
Beyond temperature monitoring, Sensaphone systems can detect equipment malfunctions, power failures and unauthorized access to provide comprehensive protection.
How Sensaphone Works
Temperature sensors are installed inside refrigerators to measure temperature levels. These sensors continuously collect data and send it to a connected Sensaphone device which displays real-time temperature readings and records them for ongoing monitoring.
If temperatures fall outside of the preset range, the remote monitoring system sends alerts via email, phone call, text or push notification so staff can quickly address issues before they compromise Semaglutide storage temperature.
Sensor readings are logged to create detailed records supporting trend analysis and regulatory compliance reporting. Cloud-based Sensaphone systems provide secure remote access, ensuring data is safely stored offsite to prevent loss. Many models include battery backup to allow continuous monitoring during power outages as an added layer of protection.
Monitor Multiple Refrigerators with One Sensaphone
Most Sensaphone systems have multiple sensor inputs enabling a single device to monitor several refrigerators simultaneously. The Sensaphone Sentinel Cloud-Based Monitoring System monitors up to 12 sensors and enables operators to track facilities remotely using a simple web-based interface.
Each refrigeration unit has its own temperature sensor, all connected to a centralized monitoring system. Independent thresholds and alert settings may be set for each sensor, ensuring precise monitoring for different storage requirements.
Sensaphone devices deliver accurate 24/7 monitoring of Semaglutide storage temperature and that of other temperature-sensitive injectables, protecting product integrity, safeguarding expensive inventory and supporting compliance for healthcare providers.
Learn more about Sensaphone pharmaceutical temperature-monitoring or contact a sales team member to discuss your application at: https://sensaphone.com/contact-sales-team/